- Arbitration
- Banking & Finance
- Capital Markets
- Commercial
- Competition
- Construction & Infrastructure
- Corporate / Mergers & Acquisitions
- Corporate Services
- Corporate Structuring
- Digital & Data
- Dispute Resolution
- Employment & Incentives
- Family Business & Private Wealth
- Innovation, Patents & Industrial Property (3IP)
- Insurance
Find a Lawyer
Book an appointment with us, or search the directory to find the right lawyer for you directly through the app.
Find out more
Real Estate & Construction and Hotels & Leisure
Real estate, construction, and hospitality are at the forefront of transformation across the Middle East – reshaping cities, driving investment, and demanding increasingly sophisticated legal frameworks.
In the June edition of Law Update, we take a closer look at the legal shifts influencing the sector – from Dubai’s new Real Estate Investment Funds Law and major reforms in Qatar, to Bahrain’s push toward digitalisation in property and timeshare regulation. We also explore practical issues around strata, zoning, joint ventures, and hotel management agreements that are critical to navigating today’s market.
As the landscape becomes more complex, understanding the legal dynamics behind these developments is key to making informed, strategic decisions.


2025 is set to be a game-changer for the MENA region, with legal and regulatory shifts from 2024 continuing to reshape its economic landscape. Saudi Arabia, the UAE, Egypt, Iraq, Qatar, and Bahrain are all implementing groundbreaking reforms in sustainable financing, investment laws, labor regulations, and dispute resolution. As the region positions itself for deeper global integration, businesses must adapt to a rapidly evolving legal environment.
Our Eyes on 2025 publication provides essential insights and practical guidance on the key legal updates shaping the year ahead—equipping you with the knowledge to stay ahead in this dynamic market.
The leading law firm in the Middle East & North Africa region.
A complete spectrum of legal services across jurisdictions in the Middle East & North Africa.
-
Practices
- All Practices
- Banking & Finance
- Capital Markets
- Commercial
- Competition
- Construction & Infrastructure
- Corporate / Mergers & Acquisitions
- Corporate Services
- Corporate Structuring
-
Sectors
-
Country Groups
-
Client Solutions
Today's news and tomorrow's trends from around the region.
17 offices across the Middle East & North Africa.
Our Services
 Back
Back
-
Practices
- All Practices
- Banking & Finance
- Capital Markets
- Commercial
- Competition
- Construction & Infrastructure
- Corporate / Mergers & Acquisitions
- Corporate Services
- Corporate Structuring
- Digital & Data
- Dispute Resolution
- Employment & Incentives
- Family Business & Private Wealth
- Innovation, Patents & Industrial Property (3IP)
- Insurance
- Intellectual Property
- Legislative Drafting
- Private Client Services
- Private Equity
- Private Notary
- Projects
- Real Estate
- Regulatory
- Tax
- Turnaround, Restructuring & Insolvency
- Compliance, Investigations and White-Collar Crime
-
Sectors
-
Country Groups
-
Client Solutions
- Law Firm
- /
- Insights
- /
- Law Update
- /
- When Healthcare & Life Sciences Imitate Art
- /
- The 2021 Heartbeat of Healthcare in the Middle East
The 2021 Heartbeat of Healthcare in the Middle East
Christina Sochacki - Senior Counsel - Corporate / Mergers and Acquisitions

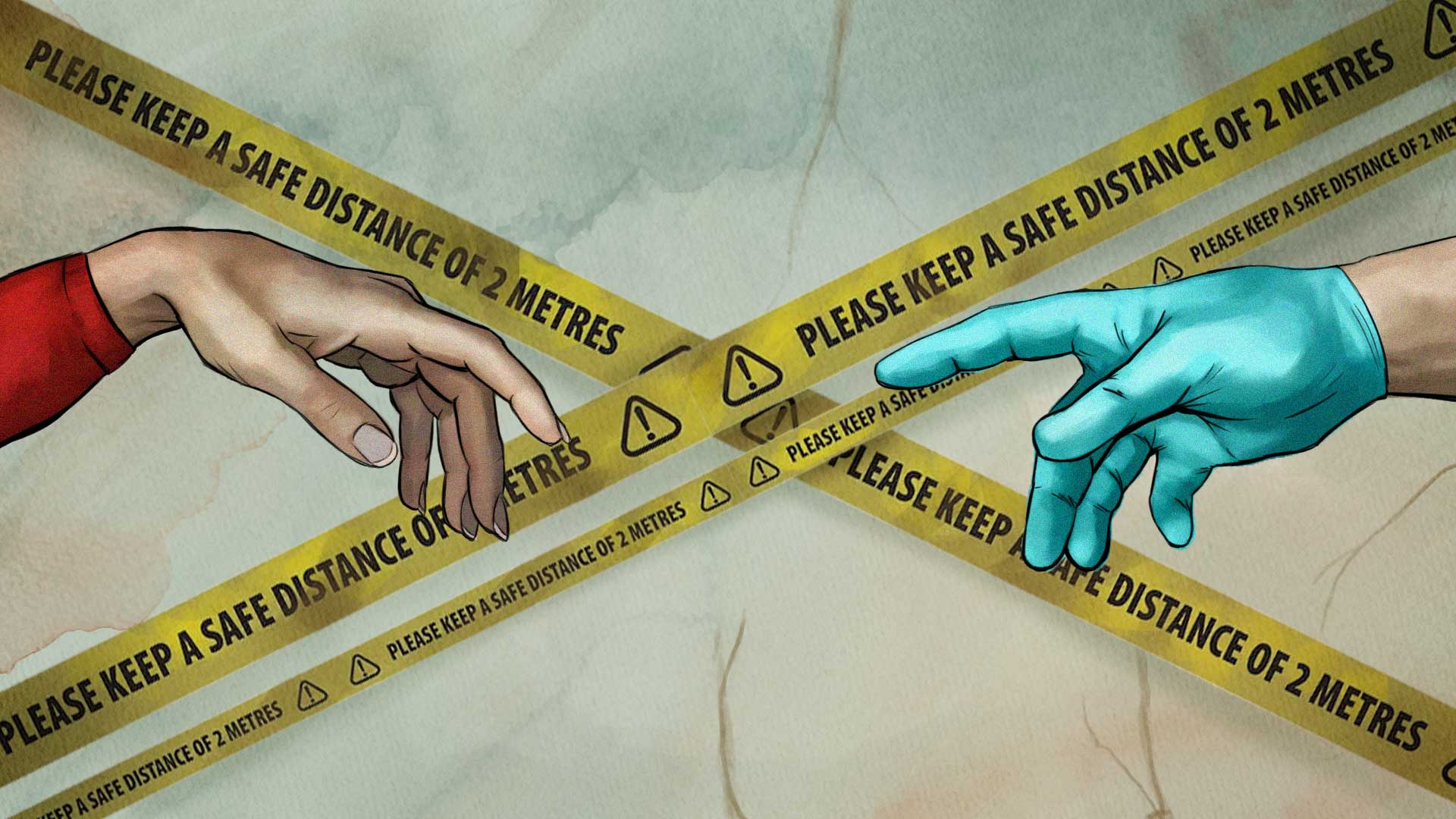
This illustration is inspired by the original painting of The Creation of Adam by Michelangelo
This healthcare and life sciences edition of Law Update examines the developments over the past year in the industries across the Middle East and North Africa. Amidst the continued plethora of COVID-19 related legislation, the regulators have continued to advance their healthcare transformation strategies. Our healthcare and life sciences sector experts remain exceptionally busy, not only supporting our clients’ understanding of COVID-19 related regulations but also the broader suite of regulatory change as the region continues to expand healthcare provision and the adoption of digital health solutions.
This edition contains 12 sector specific articles covering a wide range of topics impacting the healthcare and life sciences industries across the region. In the United Arab Emirates (‘UAE’), we have observed another busy period of regulatory output. As companies seek to have their employees return to working in an office, the question of COVID-19 vaccines and employers obligations to ensure the health and safety of their employees is a hot topic. We address the state of play in the UAE, the Kingdom of Saudi Arabia (‘KSA’) and State of Qatar (see ‘COVID/Vaccines: The New Normal: Return to Work Arrangements’ here).
In an effort to advance the healthcare system in the Emirate of Dubai, the Dubai Health Authority (‘DHA’) has been bifurcated into the DHA and the Dubai Academic Health Corporation. We dive into what this means in our article ‘Establishment of the Dubai Academic Health Corporation’ (here). On the whole, the DHA regulatory arm was extremely active; we set out the key Dubai healthcare regulatory developments of 2021 in our similarly titled article (see ‘Key Dubai Healthcare Regulatory Developments of 2021’, here). Following last year’s Ministry of Health & Prevention’s executive regulations to the federal law on information communication technology in healthcare, a new resolution was passed detailing the exemptions to the restriction on the transfer of health data outside of the UAE (see ‘Setting Health Boundaries’, here). As vaccines have been a focal point for many regulators these last few years, we discuss vaccine patents and their legal framework in the UAE (see ‘Vaccine Patents and Legal Framework in the UAE’, here).
Further, the Emirate of Sharjah’s health authority established the Sharjah Healthcare City free zone, implementing its regulatory framework last year (read more here). On the litigation front, we examine a health facility’s liability for the safety of medical equipment, based on a recent landmark judgment issued in favour of one of Al Tamimi & Company’s health facility clients (read more here). When a court assess damages payable to a patient after a medical error has occurred in the UAE, whether a patient obtained subsequent medical care through their insurance coverage will impact the amount of damages awarded, as elaborated in our judgement summary ‘UAE Judgment – The assessment of damages payable to injured healthcare patients’ (here).
In KSA, the Saudi Food & Drug Authority (‘SFDA’) issued new guidance on artificial intelligence and big data medical devices, which follows Saudi’s efforts to ensure the adoption innovative technology for the delivery of healthcare services in the Kingdom (see ‘New Guidance for AI and Big Data Medical Devices in Saudi Arabia’ here). When it comes to medical devices and pharmaceutical, in 2021 there was a deluge of updates to these regulatory frameworks, including a long awaited new medical device law to supersede the interim regulations; we highlight the key medical device and pharmaceutical laws and regulatory developments of the past year in our article ‘Key Saudi Medical Device and Pharmaceutical Laws’ (here).
In the past decade, multiple nations in the MENA region have increased their focus on enhancing scientific capabilities in order to tap into the prospects and benefits associated with stem-cell research. Our Bahrain team provides a high-level overview of some of the key legislative developments in the field of stem cell research in Bahrain (read more here).
Lastly, in recent years, the region has been projected to be one of the fastest growing markets for clinical research, due to availability of the required infrastructure, access to patients, faster timelines and lower costs compared to other markets. As the second-biggest destination country for clinical trials in Africa, Egypt has witnessed a steady increase in the number of trials it hosts. The Egyptian Parliament approved a clinical medical research bill at the end of 2020, considered to be the first unified legal framework regulating medical research (read more here).
We hope that you enjoy this special edition of Law Update. Al Tamimi & Company’s specialist healthcare and life sciences lawyers across our 16 offices in nine jurisdictions regularly advise on legal and regulatory matters concerning the healthcare and life sciences sectors.
For more details on our offering and how we can assist you, please contact us at healthcare@tamimi.com.
Stay updated
To learn more about our services and get the latest legal insights from across the Middle East and North Africa region, click on the link below.


